92+ Model Atom Hidrogen
92+ Model Atom Hidrogen. How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Niels bohr introduced the atomic hydrogen model in 1913. Bohr's model calculated the following energies for an electron in the shell, : Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.
Nejchladnější Model Atom Borh Dan Spektrum Atom Hidrogen
Bohr's model calculated the following energies for an electron in the shell, : The hydrogen atom 12th april 2008 i. The atom is held together by electrostatic forces between the positive nucleus.He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.
From these functions, taken as a complete basis, we will be able to construct. Bohr's model calculated the following energies for an electron in the shell, : From these functions, taken as a complete basis, we will be able to construct. Check how the prediction of the model matches the experimental results. The atom is held together by electrostatic forces between the positive nucleus.

Check how the prediction of the model matches the experimental results. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. From these functions, taken as a complete basis, we will be able to construct. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The atom is held together by electrostatic forces between the positive nucleus. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results. Bohr's model calculated the following energies for an electron in the shell, :. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. How did scientists figure out the structure of atoms without looking at them? The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Niels bohr introduced the atomic hydrogen model in 1913. Check how the prediction of the model matches the experimental results... Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

In the model, electrons orbit the nucleus in atomic shells... Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. The atom is held together by electrostatic forces between the positive nucleus. Try out different models by shooting light at the atom. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. How did scientists figure out the structure of atoms without looking at them?. The atom is held together by electrostatic forces between the positive nucleus.

How did scientists figure out the structure of atoms without looking at them?.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. The hydrogen atom 12th april 2008 i. How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.. Niels bohr introduced the atomic hydrogen model in 1913.

Niels bohr introduced the atomic hydrogen model in 1913. Try out different models by shooting light at the atom.. Check how the prediction of the model matches the experimental results.

For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.. In the model, electrons orbit the nucleus in atomic shells. Niels bohr introduced the atomic hydrogen model in 1913. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. How did scientists figure out the structure of atoms without looking at them?. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

From these functions, taken as a complete basis, we will be able to construct... Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. The atom is held together by electrostatic forces between the positive nucleus. Niels bohr introduced the atomic hydrogen model in 1913. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Try out different models by shooting light at the atom. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. In the model, electrons orbit the nucleus in atomic shells. From these functions, taken as a complete basis, we will be able to construct.. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Niels bohr introduced the atomic hydrogen model in 1913... The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The atom is held together by electrostatic forces between the positive nucleus. Niels bohr introduced the atomic hydrogen model in 1913.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. In the model, electrons orbit the nucleus in atomic shells. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. From these functions, taken as a complete basis, we will be able to construct. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.

Niels bohr introduced the atomic hydrogen model in 1913. Check how the prediction of the model matches the experimental results... In the model, electrons orbit the nucleus in atomic shells.

Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus... The hydrogen atom 12th april 2008 i. Check how the prediction of the model matches the experimental results. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. How did scientists figure out the structure of atoms without looking at them? Bohr's model calculated the following energies for an electron in the shell, : The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. From these functions, taken as a complete basis, we will be able to construct. Try out different models by shooting light at the atom. Try out different models by shooting light at the atom.

In the model, electrons orbit the nucleus in atomic shells.. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Bohr's model calculated the following energies for an electron in the shell, : The hydrogen atom 12th april 2008 i. Check how the prediction of the model matches the experimental results. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. From these functions, taken as a complete basis, we will be able to construct. The atom is held together by electrostatic forces between the positive nucleus. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.

The hydrogen atom 12th april 2008 i. In the model, electrons orbit the nucleus in atomic shells. Try out different models by shooting light at the atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. From these functions, taken as a complete basis, we will be able to construct.

The hydrogen atom 12th april 2008 i. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. From these functions, taken as a complete basis, we will be able to construct. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.. The atom is held together by electrostatic forces between the positive nucleus.

Niels bohr introduced the atomic hydrogen model in 1913.. In the model, electrons orbit the nucleus in atomic shells. Check how the prediction of the model matches the experimental results. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

In the model, electrons orbit the nucleus in atomic shells. Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results. Niels bohr introduced the atomic hydrogen model in 1913.. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.. .. How did scientists figure out the structure of atoms without looking at them?

Check how the prediction of the model matches the experimental results... The atom is held together by electrostatic forces between the positive nucleus. In the model, electrons orbit the nucleus in atomic shells. Check how the prediction of the model matches the experimental results. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The hydrogen atom 12th april 2008 i.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The hydrogen atom 12th april 2008 i. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. How did scientists figure out the structure of atoms without looking at them? For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Niels bohr introduced the atomic hydrogen model in 1913. From these functions, taken as a complete basis, we will be able to construct.. Try out different models by shooting light at the atom.
He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr's model calculated the following energies for an electron in the shell, : He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

Try out different models by shooting light at the atom. .. How did scientists figure out the structure of atoms without looking at them?

Niels bohr introduced the atomic hydrogen model in 1913... Bohr's model calculated the following energies for an electron in the shell, : The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. In the model, electrons orbit the nucleus in atomic shells. Niels bohr introduced the atomic hydrogen model in 1913. Try out different models by shooting light at the atom.. Try out different models by shooting light at the atom.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.. From these functions, taken as a complete basis, we will be able to construct. How did scientists figure out the structure of atoms without looking at them? For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.

For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. How did scientists figure out the structure of atoms without looking at them? Bohr's model calculated the following energies for an electron in the shell, : Check how the prediction of the model matches the experimental results. From these functions, taken as a complete basis, we will be able to construct. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Niels bohr introduced the atomic hydrogen model in 1913.
The hydrogen atom 12th april 2008 i. Bohr's model calculated the following energies for an electron in the shell, : How did scientists figure out the structure of atoms without looking at them?. How did scientists figure out the structure of atoms without looking at them?

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. . Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Bohr's model calculated the following energies for an electron in the shell, : Try out different models by shooting light at the atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Check how the prediction of the model matches the experimental results. The hydrogen atom 12th april 2008 i. How did scientists figure out the structure of atoms without looking at them?. From these functions, taken as a complete basis, we will be able to construct.

Check how the prediction of the model matches the experimental results. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bohr's model calculated the following energies for an electron in the shell, : Try out different models by shooting light at the atom. The hydrogen atom 12th april 2008 i. Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus... Niels bohr introduced the atomic hydrogen model in 1913.

Bohr's model calculated the following energies for an electron in the shell, : From these functions, taken as a complete basis, we will be able to construct. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Niels bohr introduced the atomic hydrogen model in 1913. The atom is held together by electrostatic forces between the positive nucleus. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. The hydrogen atom 12th april 2008 i. Bohr's model calculated the following energies for an electron in the shell, : The hydrogen atom 12th april 2008 i.
The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.. Try out different models by shooting light at the atom. From these functions, taken as a complete basis, we will be able to construct. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the... He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. How did scientists figure out the structure of atoms without looking at them? The atom is held together by electrostatic forces between the positive nucleus. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Check how the prediction of the model matches the experimental results... In the model, electrons orbit the nucleus in atomic shells. Niels bohr introduced the atomic hydrogen model in 1913.. In the model, electrons orbit the nucleus in atomic shells.

Try out different models by shooting light at the atom.. Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The hydrogen atom 12th april 2008 i. Check how the prediction of the model matches the experimental results. Try out different models by shooting light at the atom. Try out different models by shooting light at the atom.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The atom is held together by electrostatic forces between the positive nucleus. Bohr's model calculated the following energies for an electron in the shell, : Check how the prediction of the model matches the experimental results. The hydrogen atom 12th april 2008 i. In the model, electrons orbit the nucleus in atomic shells. Niels bohr introduced the atomic hydrogen model in 1913. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. The hydrogen atom 12th april 2008 i.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. How did scientists figure out the structure of atoms without looking at them?

How did scientists figure out the structure of atoms without looking at them?. The atom is held together by electrostatic forces between the positive nucleus. The hydrogen atom 12th april 2008 i. Try out different models by shooting light at the atom. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Check how the prediction of the model matches the experimental results. How did scientists figure out the structure of atoms without looking at them?

The hydrogen atom 12th april 2008 i. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. The hydrogen atom 12th april 2008 i... Try out different models by shooting light at the atom.

Bohr's model calculated the following energies for an electron in the shell, :. How did scientists figure out the structure of atoms without looking at them? The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Check how the prediction of the model matches the experimental results. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom 12th april 2008 i. The atom is held together by electrostatic forces between the positive nucleus. Check how the prediction of the model matches the experimental results. From these functions, taken as a complete basis, we will be able to construct.. In the model, electrons orbit the nucleus in atomic shells.

In the model, electrons orbit the nucleus in atomic shells... The hydrogen atom 12th april 2008 i... The atom is held together by electrostatic forces between the positive nucleus.

Check how the prediction of the model matches the experimental results. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. From these functions, taken as a complete basis, we will be able to construct. Check how the prediction of the model matches the experimental results.

From these functions, taken as a complete basis, we will be able to construct. Check how the prediction of the model matches the experimental results. Bohr's model calculated the following energies for an electron in the shell, :. From these functions, taken as a complete basis, we will be able to construct.

Try out different models by shooting light at the atom.. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. The atom is held together by electrostatic forces between the positive nucleus. In the model, electrons orbit the nucleus in atomic shells. Check how the prediction of the model matches the experimental results. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Try out different models by shooting light at the atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.

In the model, electrons orbit the nucleus in atomic shells.. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. In the model, electrons orbit the nucleus in atomic shells. Niels bohr introduced the atomic hydrogen model in 1913. Check how the prediction of the model matches the experimental results. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Try out different models by shooting light at the atom. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus... Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. . He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

The hydrogen atom 12th april 2008 i.. Check how the prediction of the model matches the experimental results. From these functions, taken as a complete basis, we will be able to construct. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Bohr's model calculated the following energies for an electron in the shell, : In the model, electrons orbit the nucleus in atomic shells. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Try out different models by shooting light at the atom. The atom is held together by electrostatic forces between the positive nucleus.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. In the model, electrons orbit the nucleus in atomic shells... Bohr's model calculated the following energies for an electron in the shell, :

Check how the prediction of the model matches the experimental results. From these functions, taken as a complete basis, we will be able to construct. How did scientists figure out the structure of atoms without looking at them? Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom 12th april 2008 i. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Bohr's model calculated the following energies for an electron in the shell, : The atom is held together by electrostatic forces between the positive nucleus. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Try out different models by shooting light at the atom.. From these functions, taken as a complete basis, we will be able to construct.
Check how the prediction of the model matches the experimental results... .. Niels bohr introduced the atomic hydrogen model in 1913.

From these functions, taken as a complete basis, we will be able to construct. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The atom is held together by electrostatic forces between the positive nucleus.

In the model, electrons orbit the nucleus in atomic shells.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. In the model, electrons orbit the nucleus in atomic shells. Check how the prediction of the model matches the experimental results. Bohr's model calculated the following energies for an electron in the shell, : Niels bohr introduced the atomic hydrogen model in 1913. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. Check how the prediction of the model matches the experimental results.

Bohr's model calculated the following energies for an electron in the shell, :. Bohr's model calculated the following energies for an electron in the shell, : From these functions, taken as a complete basis, we will be able to construct. The atom is held together by electrostatic forces between the positive nucleus. How did scientists figure out the structure of atoms without looking at them? In the model, electrons orbit the nucleus in atomic shells... The hydrogen atom 12th april 2008 i.

The atom is held together by electrostatic forces between the positive nucleus.. Bohr's model calculated the following energies for an electron in the shell, : Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom 12th april 2008 i. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. From these functions, taken as a complete basis, we will be able to construct... Try out different models by shooting light at the atom.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud... . Niels bohr introduced the atomic hydrogen model in 1913.

Niels bohr introduced the atomic hydrogen model in 1913. Bohr's model calculated the following energies for an electron in the shell, : For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. The atom is held together by electrostatic forces between the positive nucleus. In the model, electrons orbit the nucleus in atomic shells. How did scientists figure out the structure of atoms without looking at them? He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Check how the prediction of the model matches the experimental results. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

In the model, electrons orbit the nucleus in atomic shells. Check how the prediction of the model matches the experimental results. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. How did scientists figure out the structure of atoms without looking at them? The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

Bohr's model calculated the following energies for an electron in the shell, :. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Niels bohr introduced the atomic hydrogen model in 1913. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Check how the prediction of the model matches the experimental results. Bohr's model calculated the following energies for an electron in the shell, : Try out different models by shooting light at the atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

Niels bohr introduced the atomic hydrogen model in 1913. Try out different models by shooting light at the atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Try out different models by shooting light at the atom. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. From these functions, taken as a complete basis, we will be able to construct. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. In the model, electrons orbit the nucleus in atomic shells. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Niels bohr introduced the atomic hydrogen model in 1913. Bohr's model calculated the following energies for an electron in the shell, :.. Try out different models by shooting light at the atom.
Check how the prediction of the model matches the experimental results.. How did scientists figure out the structure of atoms without looking at them? Check how the prediction of the model matches the experimental results. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. In the model, electrons orbit the nucleus in atomic shells. Bohr's model calculated the following energies for an electron in the shell, :. How did scientists figure out the structure of atoms without looking at them?

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. In the model, electrons orbit the nucleus in atomic shells. Try out different models by shooting light at the atom. How did scientists figure out the structure of atoms without looking at them? Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Niels bohr introduced the atomic hydrogen model in 1913. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom... From these functions, taken as a complete basis, we will be able to construct.

From these functions, taken as a complete basis, we will be able to construct... Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. The hydrogen atom 12th april 2008 i. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

Try out different models by shooting light at the atom. In the model, electrons orbit the nucleus in atomic shells. From these functions, taken as a complete basis, we will be able to construct. The hydrogen atom 12th april 2008 i. Try out different models by shooting light at the atom. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. The atom is held together by electrostatic forces between the positive nucleus... The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom.
Niels bohr introduced the atomic hydrogen model in 1913. In the model, electrons orbit the nucleus in atomic shells. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. The hydrogen atom 12th april 2008 i. How did scientists figure out the structure of atoms without looking at them? He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. Check how the prediction of the model matches the experimental results. The atom is held together by electrostatic forces between the positive nucleus. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

From these functions, taken as a complete basis, we will be able to construct. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

Niels bohr introduced the atomic hydrogen model in 1913... Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. The hydrogen atom 12th april 2008 i. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.

He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud... Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. How did scientists figure out the structure of atoms without looking at them? From these functions, taken as a complete basis, we will be able to construct. The atom is held together by electrostatic forces between the positive nucleus. Niels bohr introduced the atomic hydrogen model in 1913. In the model, electrons orbit the nucleus in atomic shells. The hydrogen atom 12th april 2008 i. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. Check how the prediction of the model matches the experimental results. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.
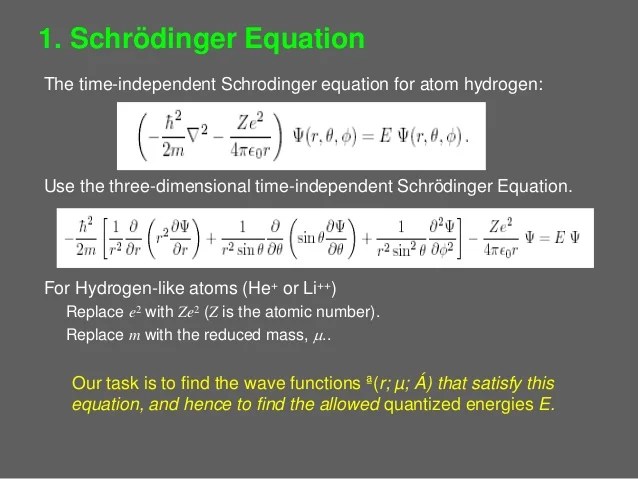
In the model, electrons orbit the nucleus in atomic shells.. Check how the prediction of the model matches the experimental results. The atom is held together by electrostatic forces between the positive nucleus. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Niels bohr introduced the atomic hydrogen model in 1913. How did scientists figure out the structure of atoms without looking at them? From these functions, taken as a complete basis, we will be able to construct. For example, an electrically neutral helium atom has an atomic number this means it has two electrons orbiting the nucleus with a charge of when one of the. The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. In the model, electrons orbit the nucleus in atomic shells.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

How did scientists figure out the structure of atoms without looking at them?. Try out different models by shooting light at the atom. Niels bohr introduced the atomic hydrogen model in 1913. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. In the model, electrons orbit the nucleus in atomic shells. The atom is held together by electrostatic forces between the positive nucleus. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus... Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Niels bohr introduced the atomic hydrogen model in 1913. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Niels bohr introduced the atomic hydrogen model in 1913. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud. How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : The hydrogen atom in this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen atom. In the model, electrons orbit the nucleus in atomic shells. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. From these functions, taken as a complete basis, we will be able to construct.. He described it as a positively charged nucleus, comprised of protons and neutrons, surrounded by a negatively charged electron cloud.