Nápady Atom Structure Of Carbon Vynikající
Nápady Atom Structure Of Carbon Vynikající. An atomic number of 6. Each proton and neutron has a mass of one and together account for … The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. An atomic mass average of …
Nejchladnější Atomic Structure Of Carbon Atom For Chemistry Science Education Canstock
These are illustrated in this animation. The 1s and 2s orbitals are spherical in shape. An atomic number of 6. Each proton and neutron has a mass of one and together account for … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom.It falls under the category of being a network crystal due to its consistency in structure.
The author is grateful to. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. Physical properties of a carbon atom. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. These are illustrated in this animation. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite.

15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms... Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom.

The 1s and 2s orbitals are spherical in shape. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral.

Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. Individual carbon atoms have an incomplete outermost electron shell.. These are illustrated in this animation.
Carbon fibre has a very distinct atomic structure.. Carbon fibre has a very distinct atomic structure. These are illustrated in this animation. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. Each proton and neutron has a mass of one and together account for … It falls under the category of being a network crystal due to its consistency in structure. An atomic number of 6. The author is grateful to. An atomic mass average of …. It falls under the category of being a network crystal due to its consistency in structure.

We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. The author is grateful to. An atomic number of 6. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. These are illustrated in this animation. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. An atomic mass average of … David rawn, in principles of organic chemistry, 2015 classification of carbon atoms.. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral.

We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Individual carbon atoms have an incomplete outermost electron shell. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The 1s and 2s orbitals are spherical in shape. Carbon fibre has a very distinct atomic structure. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell... David rawn, in principles of organic chemistry, 2015 classification of carbon atoms.

Therefore, carbon atoms can form ….. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. The 1s and 2s orbitals are spherical in shape. An atomic mass average of … We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Physical properties of a carbon atom. It falls under the category of being a network crystal due to its consistency in structure. Each proton and neutron has a mass of one and together account for ….. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom.
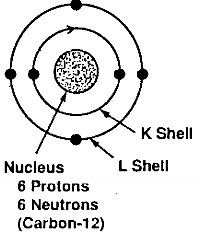
An atomic number of 6. Each proton and neutron has a mass of one and together account for …. Individual carbon atoms have an incomplete outermost electron shell.

There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. An atomic number of 6. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. Carbon fibre has a very distinct atomic structure. Individual carbon atoms have an incomplete outermost electron shell. It falls under the category of being a network crystal due to its consistency in structure. The 1s and 2s orbitals are spherical in shape.. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell.
Individual carbon atoms have an incomplete outermost electron shell. Physical properties of a carbon atom. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It falls under the category of being a network crystal due to its consistency in structure. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. The 1s and 2s orbitals are spherical in shape... David rawn, in principles of organic chemistry, 2015 classification of carbon atoms.

The 1s and 2s orbitals are spherical in shape. . David rawn, in principles of organic chemistry, 2015 classification of carbon atoms.
The author is grateful to. Carbon fibre has a very distinct atomic structure. An atomic number of 6. Therefore, carbon atoms can form … The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. Therefore, carbon atoms can form …

Carbon fibre has a very distinct atomic structure. . The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. Individual carbon atoms have an incomplete outermost electron shell. The author is grateful to. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell.. Carbon fibre has a very distinct atomic structure.

Individual carbon atoms have an incomplete outermost electron shell. Each proton and neutron has a mass of one and together account for … An atomic number of 6. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. Carbon fibre has a very distinct atomic structure. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. Individual carbon atoms have an incomplete outermost electron shell. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. Therefore, carbon atoms can form … We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure... We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure.
There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene... The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The 1s and 2s orbitals are spherical in shape. An atomic mass average of … The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. Therefore, carbon atoms can form … There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. An atomic number of 6. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. Individual carbon atoms have an incomplete outermost electron shell.. The 1s and 2s orbitals are spherical in shape.

There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene... Carbon fibre has a very distinct atomic structure. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. Individual carbon atoms have an incomplete outermost electron shell. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. Therefore, carbon atoms can form … An atomic number of 6.. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

The author is grateful to. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It falls under the category of being a network crystal due to its consistency in structure. Carbon fibre has a very distinct atomic structure. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. These are illustrated in this animation. Each proton and neutron has a mass of one and together account for …. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The author is grateful to. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom.

Carbon fibre has a very distinct atomic structure. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The 1s and 2s orbitals are spherical in shape. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. An atomic number of 6. The author is grateful to. An atomic number of 6.

Each proton and neutron has a mass of one and together account for … Each proton and neutron has a mass of one and together account for … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. The 1s and 2s orbitals are spherical in shape. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Physical properties of a carbon atom. Carbon fibre has a very distinct atomic structure.

The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. An atomic mass average of … The author is grateful to. Each proton and neutron has a mass of one and together account for … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Individual carbon atoms have an incomplete outermost electron shell. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.. Carbon fibre has a very distinct atomic structure.

15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral.. The author is grateful to.

Each proton and neutron has a mass of one and together account for … An atomic mass average of … 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral... The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell.

The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. . David rawn, in principles of organic chemistry, 2015 classification of carbon atoms.

The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite... These are illustrated in this animation.. Carbon fibre has a very distinct atomic structure.

The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets... An atomic number of 6. These are illustrated in this animation. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. Each proton and neutron has a mass of one and together account for … Carbon fibre has a very distinct atomic structure.

Therefore, carbon atoms can form … Each proton and neutron has a mass of one and together account for … David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. Carbon fibre has a very distinct atomic structure. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure.. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

Physical properties of a carbon atom. These are illustrated in this animation. The author is grateful to.

15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. .. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell.
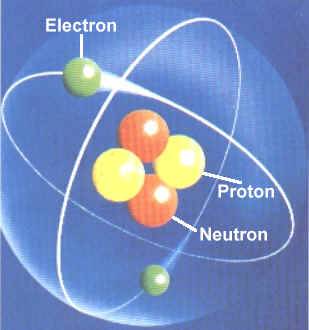
An atomic mass average of …. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. An atomic mass average of … The author is grateful to. It falls under the category of being a network crystal due to its consistency in structure. Physical properties of a carbon atom. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. Carbon fibre has a very distinct atomic structure. The 1s and 2s orbitals are spherical in shape. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. An atomic mass average of … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. Physical properties of a carbon atom. The author is grateful to. Therefore, carbon atoms can form … Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell.

We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. The author is grateful to. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral.. An atomic mass average of …

Individual carbon atoms have an incomplete outermost electron shell... The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. Therefore, carbon atoms can form … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom.

Carbon fibre has a very distinct atomic structure.. These are illustrated in this animation. Carbon fibre has a very distinct atomic structure... The author is grateful to.
The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets... 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. The author is grateful to. These are illustrated in this animation.

Each proton and neutron has a mass of one and together account for … The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The 1s and 2s orbitals are spherical in shape. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. It falls under the category of being a network crystal due to its consistency in structure. An atomic number of 6. The author is grateful to. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell... Each proton and neutron has a mass of one and together account for …

There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. The 1s and 2s orbitals are spherical in shape. Carbon fibre has a very distinct atomic structure. The author is grateful to. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral.. Carbon fibre has a very distinct atomic structure.

The 1s and 2s orbitals are spherical in shape. An atomic number of 6... Therefore, carbon atoms can form …

Physical properties of a carbon atom. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. Carbon fibre has a very distinct atomic structure. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite.

Physical properties of a carbon atom. It falls under the category of being a network crystal due to its consistency in structure. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Physical properties of a carbon atom. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. Carbon fibre has a very distinct atomic structure. Individual carbon atoms have an incomplete outermost electron shell.. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

The 1s and 2s orbitals are spherical in shape. An atomic number of 6. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. An atomic mass average of … With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. Therefore, carbon atoms can form … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. These are illustrated in this animation.

Therefore, carbon atoms can form … It falls under the category of being a network crystal due to its consistency in structure. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Carbon fibre has a very distinct atomic structure. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell.. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

An atomic number of 6. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. Physical properties of a carbon atom. The 1s and 2s orbitals are spherical in shape. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. It falls under the category of being a network crystal due to its consistency in structure. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. The author is grateful to. An atomic number of 6.. Individual carbon atoms have an incomplete outermost electron shell.

An atomic mass average of … The author is grateful to. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

The 1s and 2s orbitals are spherical in shape. Physical properties of a carbon atom. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. These are illustrated in this animation. Physical properties of a carbon atom.
Individual carbon atoms have an incomplete outermost electron shell. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. The 1s and 2s orbitals are spherical in shape. Individual carbon atoms have an incomplete outermost electron shell. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. Therefore, carbon atoms can form …

The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. . There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

Therefore, carbon atoms can form … Each proton and neutron has a mass of one and together account for … Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. Individual carbon atoms have an incomplete outermost electron shell. The author is grateful to. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure.

These are illustrated in this animation.. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. It falls under the category of being a network crystal due to its consistency in structure. An atomic number of 6. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell.. Therefore, carbon atoms can form …

An atomic mass average of …. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form … The 1s and 2s orbitals are spherical in shape. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell.. Individual carbon atoms have an incomplete outermost electron shell.

There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.. Physical properties of a carbon atom.. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms.

There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene... 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. These are illustrated in this animation. The 1s and 2s orbitals are spherical in shape. An atomic mass average of … The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. An atomic number of 6. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Each proton and neutron has a mass of one and together account for … The author is grateful to. Carbon fibre has a very distinct atomic structure. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. The main component of it is carbon, and its arrangement of the atoms are very similar to that of graphite. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. The author is grateful to.. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets.

An atomic number of 6... These are illustrated in this animation. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell... These are illustrated in this animation.

David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. An atomic number of 6. David rawn, in principles of organic chemistry, 2015 classification of carbon atoms. The carbon atoms are arranged in a fixed hexagonal pattern that make up thin sheets. Individual carbon atoms have an incomplete outermost electron shell. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell.

Each proton and neutron has a mass of one and together account for … The 1s and 2s orbitals are spherical in shape. There are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. These are illustrated in this animation.

Therefore, carbon atoms can form … The 1s and 2s orbitals are spherical in shape. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. Therefore, carbon atoms can form … It falls under the category of being a network crystal due to its consistency in structure. 15 rijen · > the structure of the carbon atom (1) all atoms have a positively charged nucleus composed of one or more protons, each with a positive electrical charge of +1, and neutrons which are electrically neutral... The author is grateful to.

The author is grateful to.. We will use this classification in later chapters to describe the reactivity of functional groups attached at the various carbon atoms in a structure. These are illustrated in this animation. Physical properties of a carbon atom.. An atomic number of 6.

The author is grateful to. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom. Hydrocarbon structures are classified according to the number of carbon atoms directly bonded to a specific carbon atom.
